Learn about the fascinating connection between sticky proteins and diabetes progression. Get insights into IAPP, beta cell dysfunction, and future diabetes cures in this comprehensive guide.
Navigating the world of diabetes can feel like piecing together a complex puzzle. We often hear about insulin, blood sugar, and lifestyle changes, but have you ever wondered about the tiny, intricate workings inside your pancreas that might play a bigger role than you think? Today, we’re diving into a fascinating and increasingly important area of diabetes research: sticky proteins in the pancreas and diabetes.
It might sound a bit sci-fi, but understanding these “sticky proteins” can shed new light on how diabetes develops and, more importantly, how we might one day prevent or even reverse it. So, let’s unpack this together, shall we?
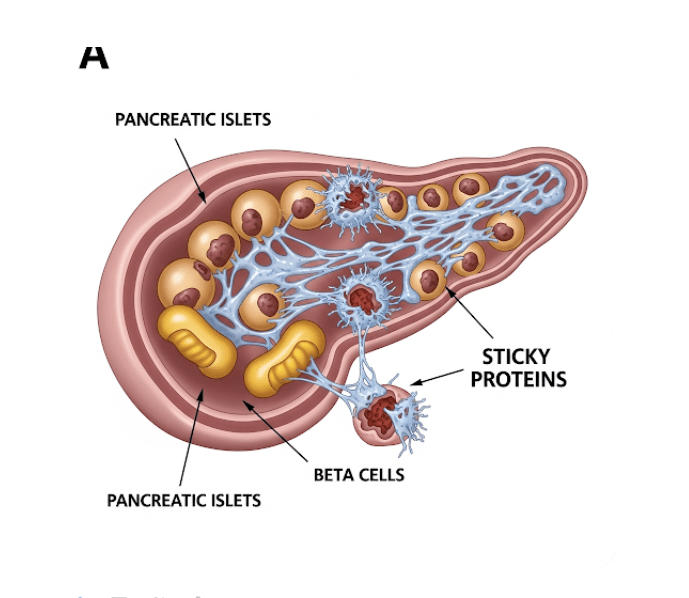
Table of Contents
What’s the Deal with Sticky Proteins in the Pancreas?
When we talk about sticky proteins in the pancreas, we’re primarily referring to a substance called islet amyloid polypeptide (IAPP), also known as amylin. Now, don’t let the fancy name intimidate you! Think of IAPP as a natural partner to insulin, both produced by the beta cells in your pancreatic islets. They’re released together in response to food, helping to regulate blood sugar.
The “sticky” part comes in when IAPP misbehaves. Under certain conditions, these IAPP molecules can clump together, forming insoluble aggregates or “amyloid fibrils.” Imagine a bunch of tiny Lego bricks that, instead of building something useful, stick together in a messy, unusable blob. These sticky clumps then accumulate within the pancreatic islets, where those all-important insulin-producing beta cells reside.
The Sticky Truth: How Do These Proteins Impact Diabetes?
Here’s where the plot thickens for people with diabetes. The accumulation of these sticky protein clumps, or amyloid deposits, is a hallmark of both type 1 and type 2 diabetes. While they’re more extensive and widespread in type 2 diabetes, they’re also present in the vast majority of individuals with type 1 diabetes.
So, what’s the connection?
Beta Cell Dysfunction: These sticky amyloid deposits are toxic to the delicate beta cells. They essentially act like tiny wrecking balls, damaging and even destroying these cells over time. This directly impacts the pancreas’s ability to produce enough insulin, leading to elevated blood sugar levels. Think of it like trying to make bread in a bakery where half the ovens are broken – you just can’t produce as much.
Inflammation and Stress: The presence of these sticky proteins can trigger an inflammatory response within the pancreas, creating a hostile environment for beta cells. This constant stress further impairs their function and survival.
Progression of Diabetes: The build-up of these sticky proteins is thought to contribute to the progressive nature of type 2 diabetes. As more and more beta cells are lost to amyloid toxicity, insulin production dwindles, making blood sugar control increasingly challenging.
Are Sticky Proteins a Cause or an Effect of Diabetes? The Chicken and Egg Question
This is a fantastic question, and one that researchers are actively exploring! It’s likely a bit of both. While the exact sequence of events is still being unraveled, evidence suggests that the formation of sticky proteins can both contribute to the development of diabetes and be exacerbated by the metabolic stresses associated with the condition.
For example, high blood sugar levels and insulin resistance, common features of prediabetes and early type 2 diabetes, can actually promote the misfolding and aggregation of IAPP. It’s a vicious cycle where one problem feeds into the other.
Can We Target These Sticky Proteins to Fight Diabetes?
This is the exciting part! Understanding the role of sticky proteins opens up entirely new avenues for potential treatments and even cures for diabetes. Imagine therapies that could:
Prevent Aggregation: Scientists are looking for ways to stop IAPP from clumping together in the first place. This could involve small molecules that bind to IAPP and keep it from misfolding, or even therapies that break down existing amyloid deposits.
Clear Existing Deposits: Research is underway to develop strategies to “clean up” the sticky protein messes that have already formed in the pancreas. Think of it like a specialized cleaning crew designed to restore your beta cells.
Protect Beta Cells: Even if some amyloid forms, therapies could be developed to shield beta cells from its toxic effects, helping them to survive and continue producing insulin.
One promising area of research involves understanding the structure of these sticky proteins at an atomic level. Companies like Amylin Pharmaceuticals (though now part of AstraZeneca) have previously explored drugs that modulate amylin activity, highlighting the therapeutic potential.
While the direct “cure” for diabetes via sticky protein intervention is still in research, numerous academic studies, often published in journals like Diabetes and Nature Medicine, are continuously advancing our understanding of this complex interplay.
What Does This Mean for You? Practical Insights
While we await groundbreaking new therapies specifically targeting sticky proteins, understanding this aspect of diabetes can still offer valuable insights:
- Lifestyle Matters More Than Ever: Factors that reduce metabolic stress, like maintaining a healthy weight, regular physical activity, and a balanced diet, can potentially reduce the likelihood of IAPP misfolding and amyloid formation. This reinforces the importance of foundational diabetes management strategies.
- Early Intervention is Key: The earlier diabetes or prediabetes is managed, the less time there is for extensive sticky protein accumulation and beta cell damage. This highlights the importance of early diagnosis and proactive care.
- Stay Informed: The world of diabetes research is constantly evolving. Keep an eye out for news and breakthroughs related to beta cell protection, islet health, and novel therapeutic targets. Reputable sources like the American Diabetes Association (diabetes.org) and the National Institute of Diabetes and Digestive and Kidney Diseases (niddk.nih.gov) are excellent resources for staying up-to-date.
The Future is Looking Less Sticky!
The journey to understanding and treating diabetes is a marathon, not a sprint. But the insights gained from studying sticky proteins in the pancreas offer a glimmer of hope for future therapies that go beyond managing symptoms.
Imagine a future where we can prevent beta cell loss, preserve insulin production, and potentially even reverse the course of diabetes by targeting these microscopic troublemakers. It’s an exciting prospect, and one that highlights the incredible complexity and resilience of the human body, and the dedication of scientists working tirelessly to unravel its mysteries.
References
Jha, A., & O’Brien, R. M. (2020). Islet amyloid polypeptide: a key player in beta-cell dysfunction in type 2 diabetes. Endocrinology, 161(3), bqaa001.
Kahn, S. E., Hull, R. L., & Verchere, K. B. (2006). The role of islet amyloid in the pathogenesis of type 2 diabetes mellitus. Journal of Molecular Endocrinology, 36(2), 241-258.
Ritzel, R. A., & Butler, P. C. (2007). Islet amyloid polypeptide: a target for type 2 diabetes therapy? Diabetologia, 50(6), 1144-1151.
